

This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other.
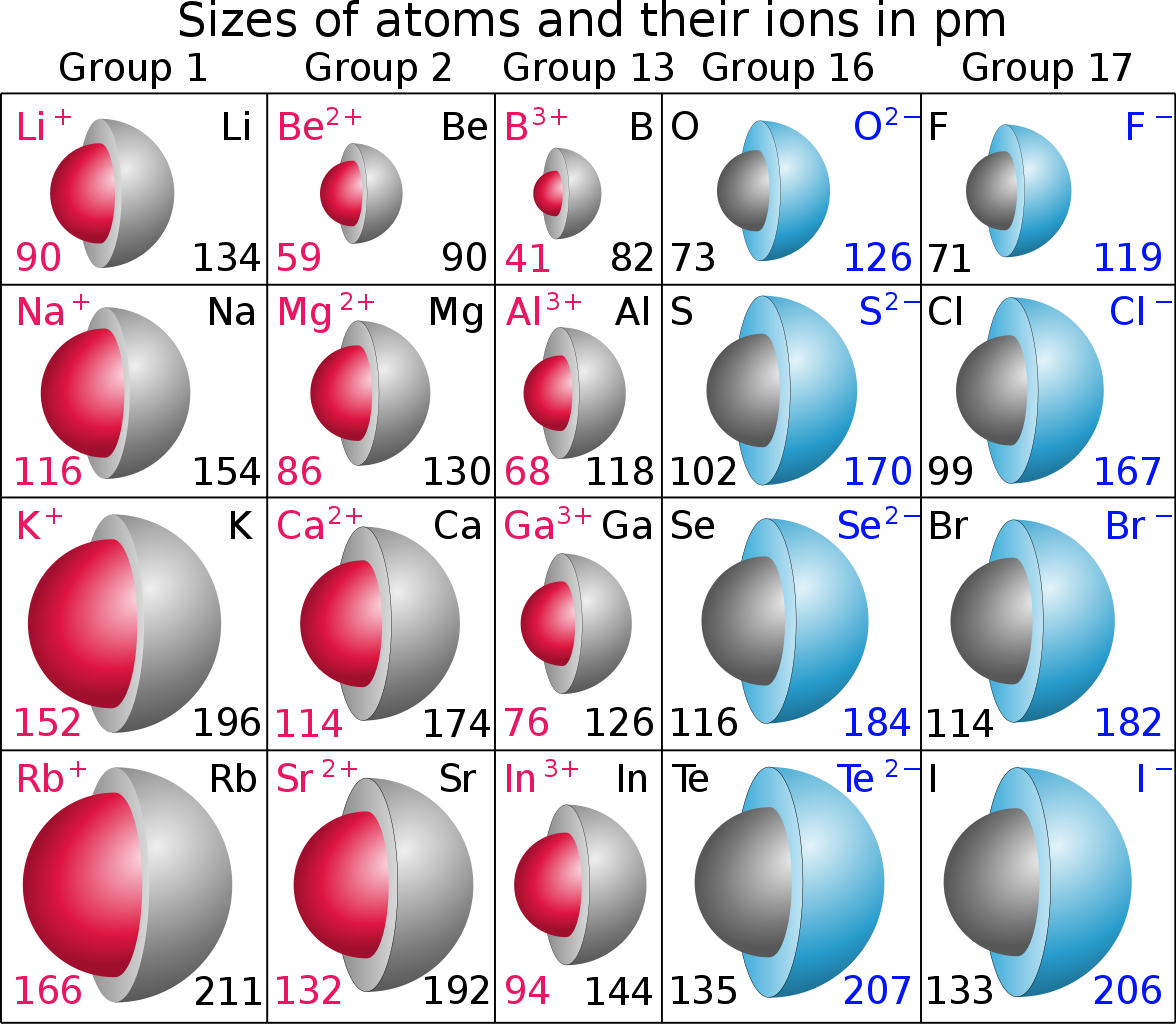
The cookies is used to store the user consent for the cookies in the category "Necessary". The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. The general explanation seems to state that as we go down a group, there are more electron shells present to contribute to a “shielding” phenomenon, where inner electrons cancel out part of the inward force from the nucleus with a repulsive force. Since the number of electron shells increase, the atom is getting larger and thus the atomic radius would get larger. sodium has 3 electron shells, potassium has 4, rubidium has 5. Why do alkali metals have large atomic radii? Explanation: Every time we move down the group, the number of electron shells of the element increases by one. Why do alkali metals have large atomic radii? 2) As you move across a period, atomic radius decreases. Therefore, the atomic radius increases as the group and energy levels increase. Each subsequent energy level is further from the nucleus than the last. – The number of energy levels increases as you move down a group as the number of electrons increases. How is the size of the atomic radius related? Down a group, the number of energy levels (n) increases, so there is a greater distance between the nucleus and the outermost orbital. In general, atomic radius decreases across a period and increases down a group. How does atomic radius decrease down a group?
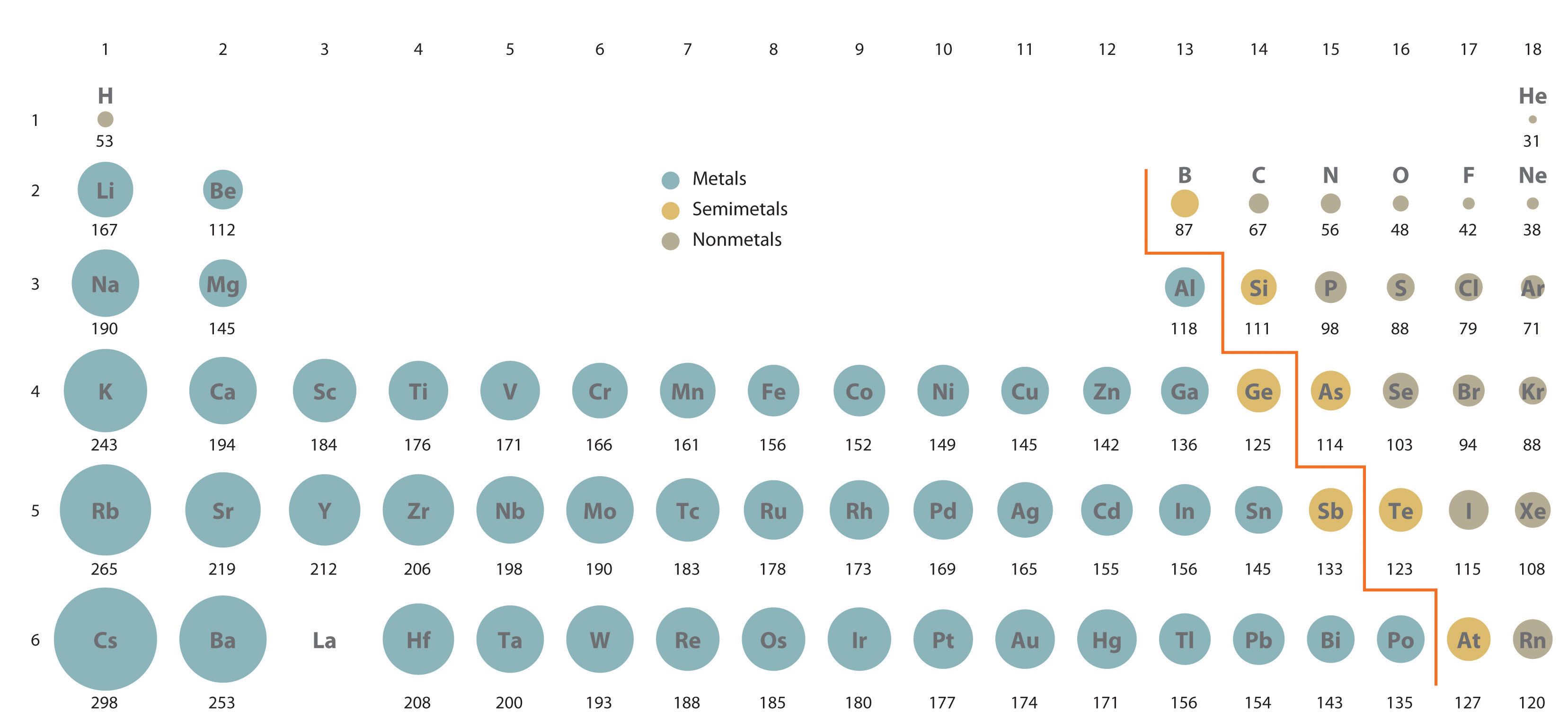
Does atomic size increase down the group? The atomic radius increases as one goes down a group in the periodic table because more electrons are around the atom and more neutons and protons are present. The new energy shells provide shielding, allowing the valence electrons to experience only a minimal amount of the protons’ positive charge. Once again protons are added moving down a group, but so are new energy shells of electrons. The atomic radius increases moving down a group. Why does the atomic radius tend to increase as you move down a group but decrease as you move across a period? When moving down a group of the periodic table, the atomic radius increases because of the presence of additional principal energy levels, which are further away from the nucleus. Why does size of atom increases down the group? Atomic radius increases from top to bottom within a group. One proton has a greater effect than one electron thus, electrons are pulled towards the nucleus, resulting in a smaller radius. This is caused by the increase in the number of protons and electrons across a period. Why does atomic radius increase from top to bottom down a group? 6 Why do alkali metals have large atomic radii?.5 How is the size of the atomic radius related?.4 Does atomic size increase down the group?.2 Why does size of atom increases down the group?.1 Why does atomic radius increase from top to bottom down a group?.Addition of another electron does not result in a fractional decrease in the electrostatic attraction to any given electron, but it does increase the electron-electron repulsion, so an overall decrease in Net attractive force. The internal energy levels “shield” and reduce electrostatic attraction of the valence electrons to the protons. (2) Number of energy levels: The greater the number of energy levels, the larger the atomic radii. (1) Nuclear charge (number of protons) : The stronger the ‘pull’ the protons have to the electrons with electrostatic attraction, then the smaller the size of the atom radii Atomic Radii is affected by two main factors :


 0 kommentar(er)
0 kommentar(er)
